PCSK9-inhibitors, like alirocumab or evolocumab, lower low-density lipoprotein cholesterol (LDL-C) by increasing the number of LDL receptors in liver cells.1,2 They have been approved by the Food and Drug Administration (FDA) for treatment of familial hypercholesterolemia and atherosclerotic cardiovascular disease, when further lipid-lowering is needed. According to the 2018 American Heart Association and American College of Cardiology guidelines, the use of PCSK9-inhibitors may be considered along with a statin or ezetimibe or both, to reach lipid-lowering goals.3 These guidelines however caution that further study of PCSK9-inhibitors is needed before the long-term safety of these medications is assured. To date, studies have been of relatively short duration and primarily designed to measure lipid-lowering efficacy.
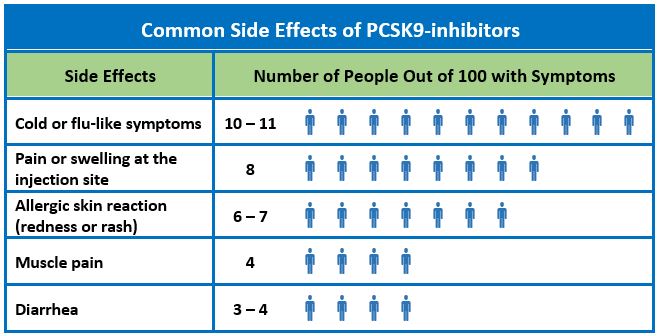
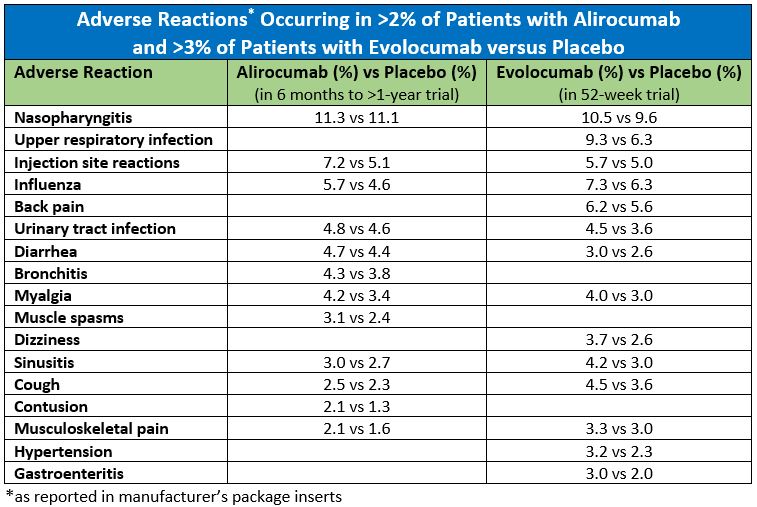
Alirocumab and evolocumab are given by subcutaneous injection. According to the manufacturer’s package inserts, after trials ranging in duration from 12 weeks to less than two years, the most common side effects are flu-like symptoms and redness, itching, or tenderness at the injection site.1,2 Allergic reactions (rash, eczema, redness, urticaria) occurred in 8.6% of patients taking alirocumab compared to 7.8% in patients taking placebo and in 5.1% of patients taking evolocumab versus 4.7% in patients taking placebo. Rarely, serious hypersensitivity reactions have occurred (frequency not specified). Other side effects that occurred with alirocumab more often than in placebo included confusion or memory impairment (0.2%) and elevated serum transaminases (1.7%). Neither statistical nor clinical significance is reported in the package inserts.
There has been some concern that the achievement of very low LDL-C levels (<25 mg/dL) as can occur taking PCSK9-inhibitors might contribute to cognitive dysfunction. Using pooled data from 14 clinical trials of alirocumab (total n=3,340 alirocumab and 1,894 control; all of two years duration or shorter), Robinson et al. determined that achieving very low LDL-C levels with alirocumab was not associated with an increase in adverse events, including reported neurocognitive events.4 Another randomized controlled trial of 1.204 patients followed for 19 months, found no significant difference between statin plus evolocumab and statin plus placebo with respect to objectively measured cognitive function.5
Sabatine et al. conducted a randomized double-blind placebo-controlled trial of evolocumab versus injected placebo (n=27,564), added to a pre-existing statin regimen.6 After a median of 26 months follow-up, with the exception of injection site reactions, there was no significant difference between the evolocumab and placebo with regard to adverse events (including new-onset diabetes, allergic reactions, and neurocognitive events).
References
- Evolocumab [package insert]. Thousand Oaks, CA: Amgen; 2017.
- Alirocumab [package insert]. Bridgewater, NJ: Sanofi-Aventis US, LLC; 2017.
- Grundy S, Stone N, Beam C, Birtcher KK, Harm PD. 2018 AHA/ACC/AACVPR/AAPA/ ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol 2019; 73 (24): e285-e350.
- Robinson J, Rosenson R, Farnier M et al. Safety of very low low-density lipoprotein cholesterol levels with alirocumab. J Am Coll Cardiol 2017; 69 (5).
- Giugliano R, Mach F, Zavitz K et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017; 377 (7): 633-643.
- Sabatine M, Gugliano R, Keech A et al. Evolocumab and clinical outcomes in patients with cardiovascualr disease. N Engl J Med 2017; 376 (18): 1713-1722.